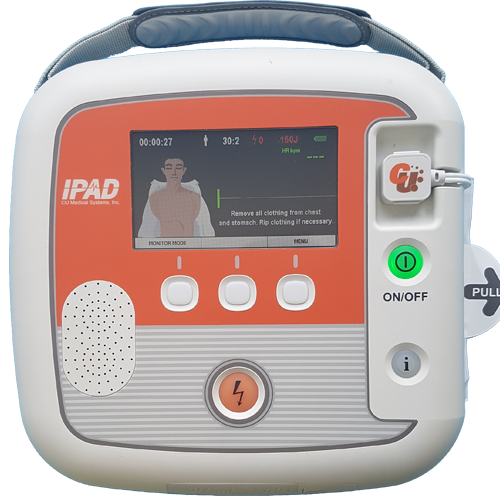
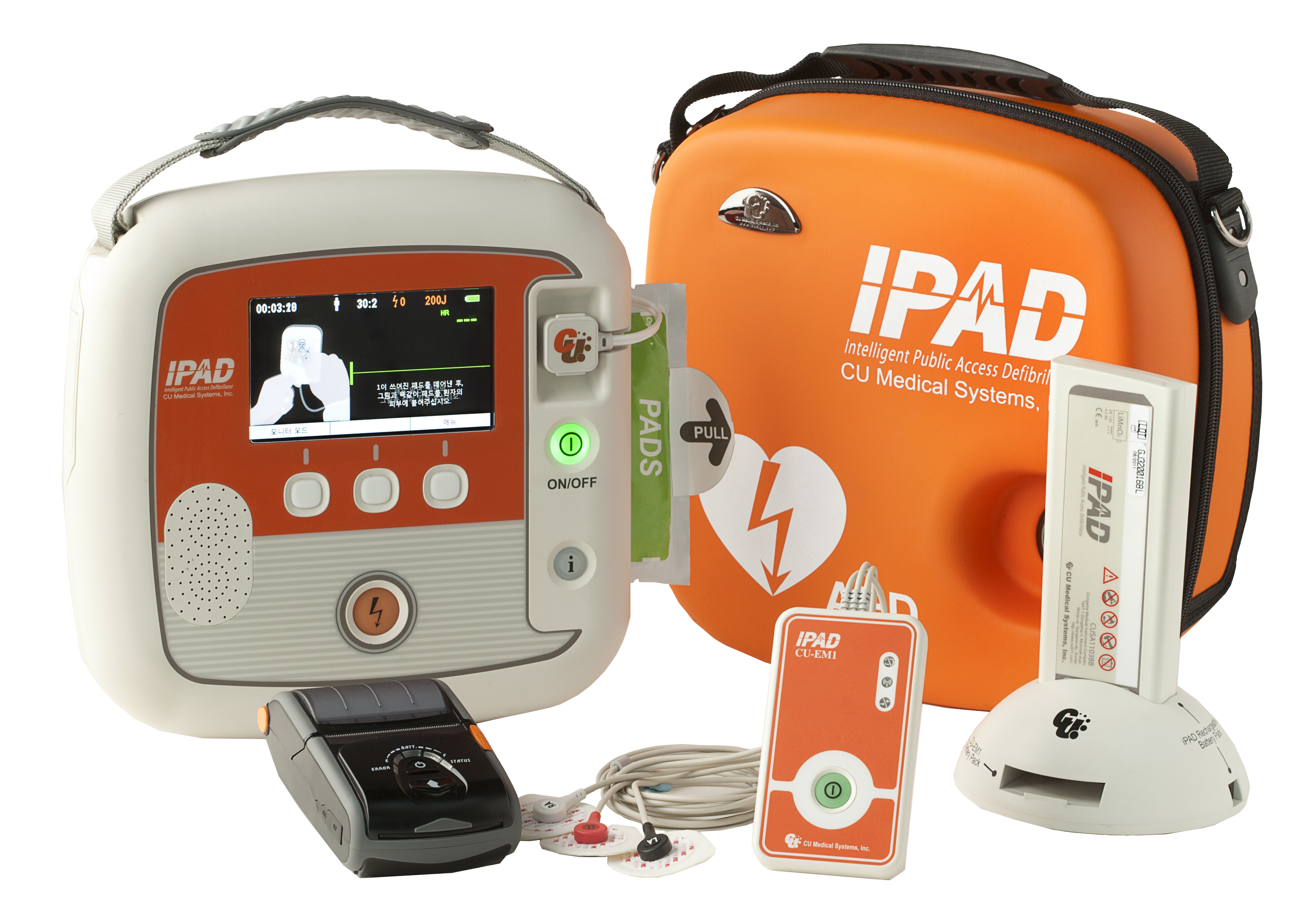
The iPAD SP2 defibrillator is designed for appropriately trained Medical Professionals who need greater functionality and clinical information than an entry level AED can provide.
Retaining the user friendliness of the SP1, the iPAD SP2 is ideal for situations where space and time are limited, but the demand for capability is not. Its lightweight, robust exterior contains a device that packs powerful functionality, required when it is needed most.